I’ve talked before about the problems series resistance [i.e. the resistance formed by the tip of your electrode and the gunk in it] poses when you’re performing whole-cell voltage clamp recordings. Simply put, it limits your ability to hold the cell at the voltages you want to, because every time you pass current, some voltage forms over the series resistance and you’re amplifier can’t tell the difference between the voltage over the membrane and the voltage over your series resistor. It also limits your temporal accuracy, as it helps to set up up a low pass filter for your command potential. Thankfully, because the problem is essentially inescapable during whole-cell voltage clamp, essentially all voltage clamp amplifiers come with series resistance compensation: an electronic procedure where the amplifier estimates how much of a problem your series resistance will be, and works to minimize it. However, in order for that circuitry to work, your amplifier needs to know the whole-cell capacitance of the cell you’re recording from (Cm) and how much series resistance (Rs) you’re up against. Typically your set these values while looking at the current response to a test pulse. However, outside of a few select examples, this process of telling the amplifier what Cm and Rs is (know as “correcting for whole-cell parameters” or “capacitance compensation”) isn’t actually trivial. Let’s have a look at why this is, and how we should be doing it in real cells
Continue readingElectrophysiology
Filtering – A practical guide
Finding good information on how filters work, what the different types of filters mean, and how you should filter your data is hard. Lots of explanations only make sense if you have a year or two of electrical engineering education, and most of the rest are just a list of rules of thumb. I want to try to get you to a place where you can test your own filter settings, and show you the importance of the rules of thumb without going into the relatively complex math that is often used to explain filters. Warning: a lot of the code I’m going to use requires the Matlab Signal Processing Toolbox. If you don’t have it, you wont be able to execute the code yourself, but hopefully you’ll still be able to follow along with the logic.
Continue reading
Electrical Stimulation: Why we use isolators.
Even though we are now in the era of optogenetics, electrical stimulation of excitable tissues is still common place in the lab. However, despite how common they are, I see that a lot of people don’t fully understand why they using some fancy expensive box to deliver the stimulus, rather than just, say, using the DAC output of your digitizer. The actual physics of why passing current through your tissue excites neurons/muscles is a bit more complex than you might think, but that’s not what I’m going to talk about. I’m going to talk about what a stimulus isolator is, and why we use them. Continue reading
The ins and outs of field potentials
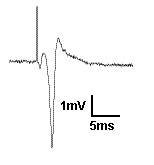
Recording field potentials was the first form of electrophysiology I ever did, and because of that, I tend to think that field potentials are simple. But the reality is far different. Field potentials, in my view, are harder to gain a solid understanding of than intracellular membrane potentials. So I’m going to try to take a ‘first principles’ approach to thinking about field potentials. To motivate us, I’m going to present exhibit ‘A’: The hippocampal CA1 population spike.
A CA1 population spike is the field potential recorded near the cell bodies of the CA1 pyramidal cells, when their afferents are stimulated strongly enough to cause the CA1 cells to spike. You see a brief upward deflection, which is an artefact from the electrical stimulation used to activate the afferents. Then you see a small downward deflection, which is due to the action potential in the afferent fibers. Then you see the population spike: the large, brief, negative potential caused by thousands of neurons spiking synchronously. It is negative because when the action potential is generated, Na+ moves into the cell, making the extracellular space negative. But here is a question: Why does the population spike appear to be resting on a positive going hump? Read on and find out, and learn more about field potentials in general.Continue reading
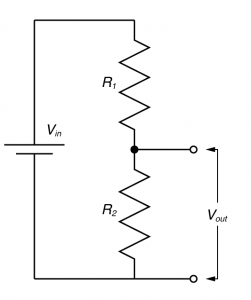
You need to understand the voltage divider!
In electronic design, the voltage divider is probably the most fundamental circuit motif. You would be hard pressed to find single circuit that doesn’t have one. But more importantly, it is a deeply useful concept for explaining the physiology of excitable cells, and for understanding the nature of electrophysiological techniques. I’ve talked about voltage dividers in several of my posts, but one of my readers said I should explain what they are so that’s what I’m going to do now.
Series Resistance. Why it’s bad.
At the very start of my Masters, my first experiments appeared to show that the histamine H3 receptor inhibited the release of GABA in the neocortex. It turns out, this was all lies. It was all lies because of series resistance, a concept I had vaguely heard of, but didn’t understand. If you’re just starting electrophysiology this post is for you. The hope is that by the end of this post, you will understand series resistance, and you’ll understand why it is extremely important to monitor it religiously, whether you’re performing voltage clamp or current clamp recordings.
Continue reading